- Industry: Healthcare
- Tools and Technologies: Python, Machine Learning, Deep Learning e Natural Language Processing
Our client
The client is a pharmaceutical startup in healthcare field, aimed at developing Integrated AI-based Pharmacovigilance Services.
Challenge
To develop a pharmacovigilance software solution to automate adverse event (AE) processing or signal detection, which can perform the processes more efficiently than manual intervention, bringing significant benefits in balancing the benefit-risk profile of the drug.
Goals:
- Optimize pharmacovigilance strategy with artificial intelligence
- Meet regulatory compliance on time (Improved compliance time by automation of manual work)
- Value addition through improved benefit-risk profiles
- Make faster and efficient safety decisions through intelligent analytics
Solution
The pharmacovigilance cycle starts with AE data collection from various sources and formats. AE case processing activities (Data Entry, Triage, QC) are performed; MedDRA Coding, ontology-based processing, and severity assessment is done.
Also, text processing like Entity Extraction, Pattern & Relation extraction, Text classification is done on data collected from literature, social media, blogs, forums etc. This process is followed by signal detection and signal management. In next step causality assessment is performed and further action has been suggestion based on statistical association measures and priority.
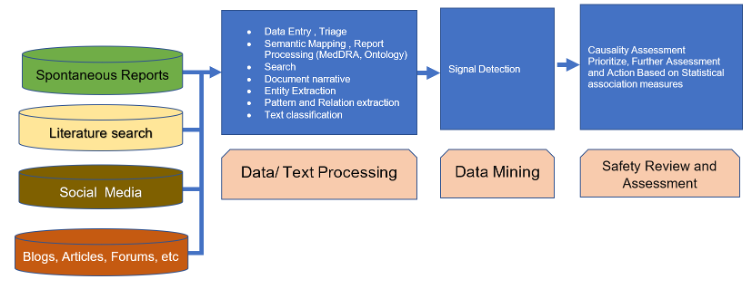
Data Analytics using Machine Language, Deep learning and Natural Language Processing from unstructured and semi-structured sources enabling predictive analytics and improved actionable insight generation to improve safety outcomes.
Natural Language Processing capabilities helps to process large texts for automatic scientific literature review and social media text. Apart from using analytics for structured data from various sources, NLP can help to processes large volumes of unstructured text to identify adverse events from various therapeutic reports, establish drug-event associations and report suspected new cases. Also, communication technologies empower us to take advantage of Internet, social media and mobile platforms to enables pharma companies proactively reach out to various stakeholder by providing easy platform (mobile app) to give feedback or complaint about the product.
Resources employed and project duration
This project employed 12 people and 2 years of time.
Result
Development of end-to-end Pharmacovigilance software, with features:
- Tool development for literature review of medical and scientific publications
- Spontaneous reporting tool for suspected Adverse Drug Reactions
Tool for signal detection & Evaluation
- Set-up and management of in-house safety database
- A systematic literature review of medical and scientific publications, social media / text mining for collection of ADR
Benefits and value additions
- Time and Cost Saving
- Improved Decision Making
- Boost Productivity
Improve Benefit-Risk profile by combining diverse data
Managing Risk proactively using predictive power
- Optimising PV landscape demanding significantly more robust solution and specialized skills
In the highly-regulated environment, it is very important to continue product on market by ensuring that benefit-risks profile is continuously tracked kept in balance








